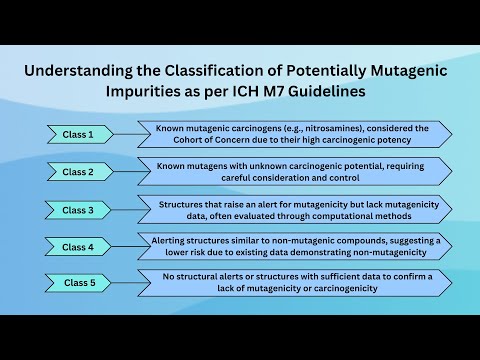
Understanding the Classification of Potentially Mutagenic Impurities as per ICH M7 Guidelines
NITROSAMINE NDSRI IMPURITY BASIC and LIMITSПодробнее

[Multidisciplinary] ICH M7 AddendumПодробнее
![[Multidisciplinary] ICH M7 Addendum](https://img.youtube.com/vi/XKnx0PRevpI/0.jpg)
Application of (Q)SAR and Expert Knowledge for ICH M7 Impurity ClassificationПодробнее

ICH M7 - Risk assessment for mutagenic impurities and control strategiesПодробнее
Understanding concepts of ICH M7 (R1) Part 1Подробнее

Risk assessment of potentially mutagenic impurities in drug products approved in BrazilПодробнее

Mutagenic Impurities from a Drug Substance Perspective: Highlights from ICH M7 Q&A Draft DocumentПодробнее

Applying Expert Knowledge for ICH M7 Impurity Classification - Session 1Подробнее

Acceptable Intakes for Mutagenic Impurities in Relation to LTL ExposureПодробнее

ICH M7(R1) – Chemistry and Manufacturing Control (CMC) Perspective on Hazard AssessmentПодробнее

What is meant by Genotoxicity & Mutagenicity?Подробнее

Applying Expert Knowledge for ICH M7 Classification - Session 2Подробнее

Application of (Q)SAR and Expert Knowledge for ICH M7 Impurity ClassificationПодробнее

Assessing the Impact of Expert Knowledge on ICH M7 (Q)SAR PredictionsПодробнее

Calculating limits for carcinogens: AI, PDE, and less than lifetime as per ICH M7Подробнее
Identified Impurity,Unidentified Impurity, Specified Impurity , Unspecified Impurity as per ICH Q3AПодробнее
[Multidisciplinary] M7(R2) / Q&As_ENGПодробнее
![[Multidisciplinary] M7(R2) / Q&As_ENG](https://img.youtube.com/vi/xPkj7HzAL_s/0.jpg)
Introduction to Using (Q)SAR for the Assessment of Potential Mutagenicity of Drug ImpuritiesПодробнее

Impurities in Drug Substances/Products: Global Guidances & USP PerspectiveПодробнее
